By Stacy M. Brown, NNPA Newswire Senior National Correspondent
The U.S. Food and Drug Administration (FDA) has greenlit two revolutionary cell-based gene therapies, Casgevy and Lyfgenia, marking a significant leap forward in treating sickle cell disease (SCD) for patients aged 12 and older.
The approval by the FDA signifies the commencement of a novel epoch in managing sickle cell disease, providing optimism to individuals whose lives have been significantly disrupted by the arduous condition.
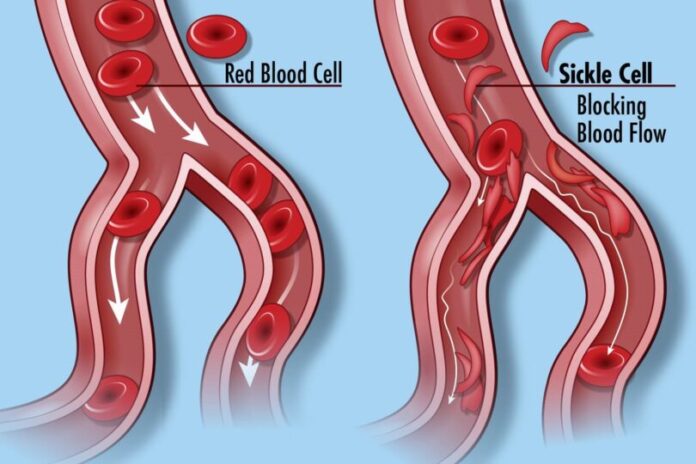
Sickle cell disease, a group of inherited blood disorders, affects around 100,000 individuals in the United States and is predominant among African Americans. Health officials said the root cause of SCD is a mutation affecting hemoglobin, a crucial protein in red blood cells responsible for oxygen delivery. The genetic problem causes red blood cells to have a unique “sickle” shape, which can lead to vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs), which are very painful and damage organs. The recurrence of these crises poses life-threatening risks and potential disabilities.
“Sickle cell disease is a rare, debilitating, and life-threatening blood disorder with significant unmet need, and we are excited to advance the field,” said Nicole Verdun, M.D., director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research.
Casgevy, a groundbreaking cell-based gene therapy, is the first FDA-approved treatment employing CRISPR/Cas9, a revolutionary genome editing technology. The therapy is for individuals 12 years of age or older who have recurrent vaso-occlusive crises. It changes the patient’s hematopoietic stem cells using CRISPR/Cas9, a technology that can precisely edit DNA.
The edited cells are then transplanted back into the patient, enhancing the production of fetal hemoglobin and preventing the sickling of red blood cells.
Lyfgenia is another cell-based gene therapy that uses a lentiviral vector to change genes. The FDA approved it for those 12 years of age or older who have SCD and a history of vaso-occlusive events. Lyfgenia changes blood stem cells to make HbAT87Q, gene-therapy-derived hemoglobin that looks like adult hemoglobin and makes it less likely that red blood cells will sickle. Both therapies utilize the patients’ blood stem cells, administered through a one-time, single-dose infusion following myeloablative conditioning.
“These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
The Casgevy and Lyfgenia applications received Priority Review, Orphan Drug, Fast Track, and Regenerative Medicine Advanced Therapy designations. Casgevy was granted approval to Vertex Pharmaceuticals, Inc., and Lyfgenia to Bluebird Bio, Inc.
The FDA said its approval of Casgevy was based on a single-arm, multicenter trial evaluating its safety and effectiveness in adult and adolescent SCD patients. Of the 44 treated patients, 93.5% achieved freedom from severe VOC episodes for at least 12 consecutive months. Common side effects included low platelet and white blood cell levels, mouth sores, nausea, and musculoskeletal pain.
Lyfgenia’s approval was based on a 24-month multicenter study, with 88% of patients achieving complete resolution of VOEs between 6- and 18-months post-infusion. Side effects included stomatitis, low blood cell levels, and febrile neutropenia. A black box warning highlighting the risk of hematologic malignancy accompanies Lyfgenia’s label, emphasizing the need for lifelong monitoring in patients.
“Today’s actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDA’s commitment to facilitating the development of safe and effective treatments for conditions with severe impacts on human health,” Dr. Marks asserted.


